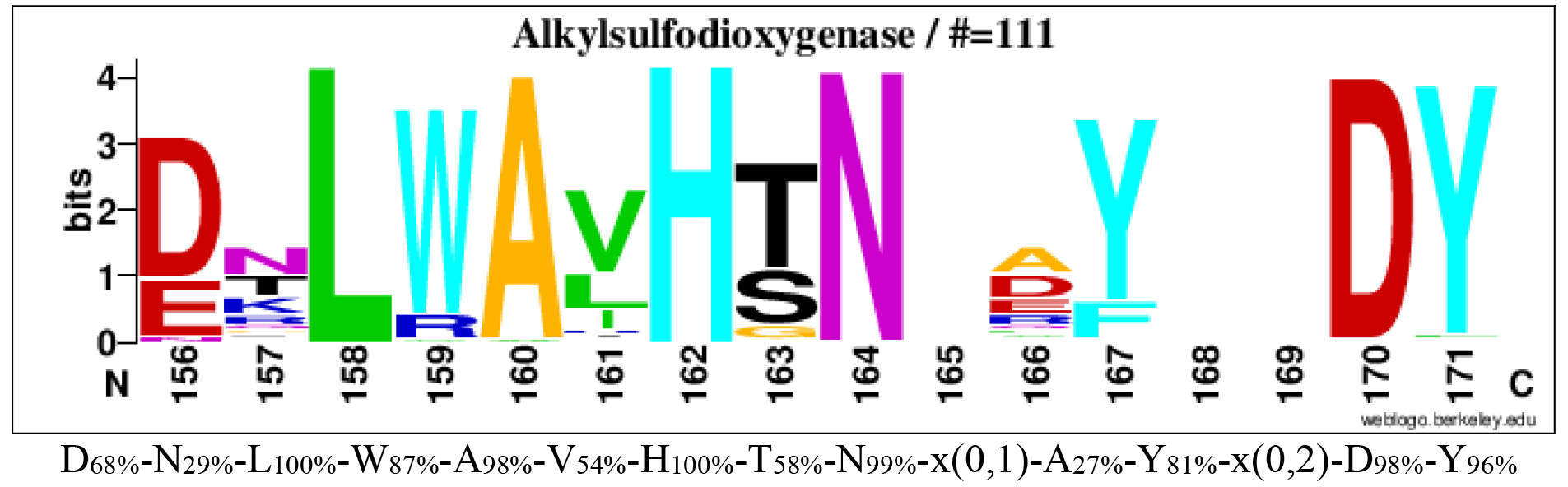Sulfatase family S2
Family S2
sulfatases belong to the non-heme iron(II)
alpha-ketoglutarate-dependent dioxygenase superfamily. They catalyze
the oxygenolytic cleavage of a variety of different alkyl sulfate
esters to the corresponding aldehyde and sulfate. The alkylsufatase
AtsK from Pseudomonas putida
S-313 is the first Family S2 sulfatases biochemically characterized.
It decarboxylates one molecule of alpha-ketoglutarate (alpha-KG) into
succinate per molecule of sulfate ester that is cleaved (Kahnert and
Kertesz, 2000). The crystal structure of this enzyme revealed a jelly
roll fold similar to the other known Fe, alpha-KG-dependent
dioxygenases (Muller et al., 2004).
Subfamilies (1)
EC activities found in subfamilies
| Subfamily |
EC Number(s) |
Description |
PubMed IDs |
The consensus sequence logoplot of family S2 (Barbeyron et al. 2016)
Logos of conserved consensus sequences identified in the global alignment of alkylsulfodioxygenases (family S2).
Logos sequences identified from aligned 111 alkylsulfodioxygenases. The numbers below the logo at the first position indicate the corresponding position in the reference sequence Atsk (Q9WWU5). The corresponding consensus sequences in multi-alignments are shown below the logo sequences. The percentages in subscript are the percentages of sequences where the amino acid is conserved in alignments. Amino acids involved in sulfate binding are in bold.
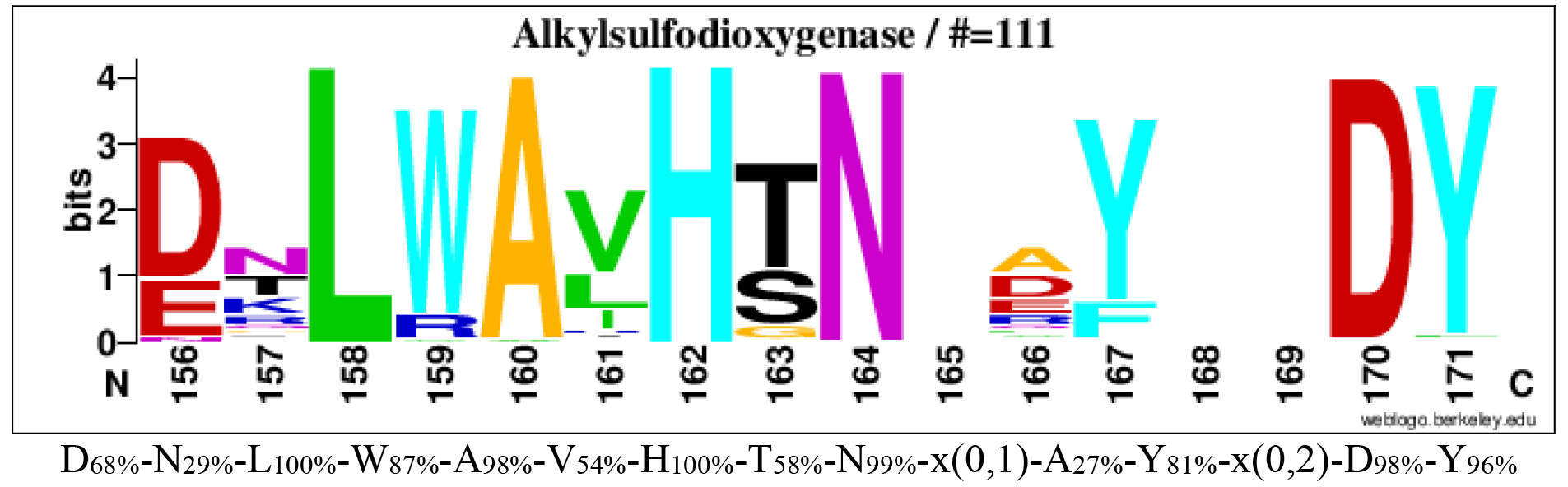
Fold and active site of a S2 family representative
Fold (A) and active site (B) of the alkylsulfatase AtsK from Pseudomonas putida S-313 (PDB code: 1OIK). The fold is shown in cartoon representation. The amino acids and ligands of the active site is shown in sticks. The cations are shown as spheres. The figures were made using PyMoL (Version 1.8 Schrödinger, LLC).
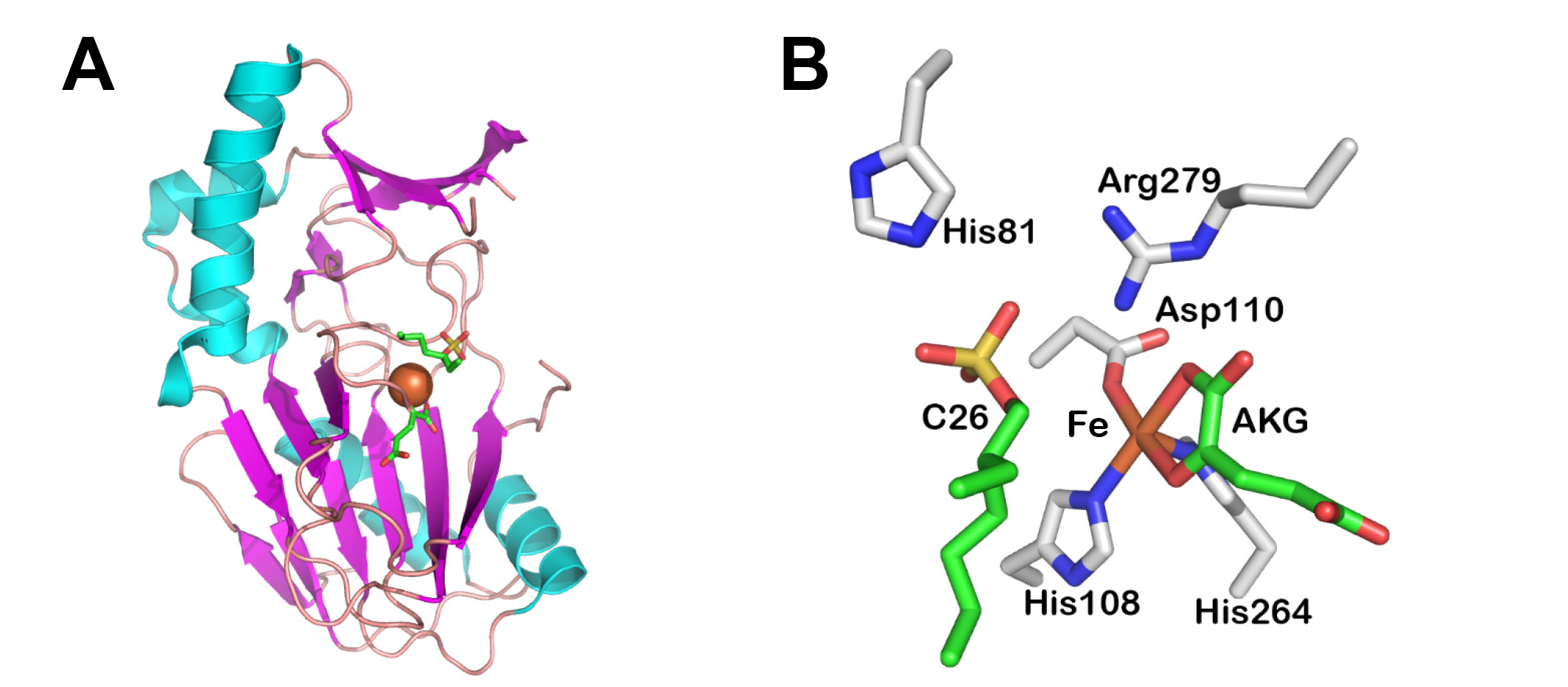
Catalytic mechanism of the S2 family sulfatases.
The numbering corresponds to the alkylsulfatase AtsK from Pseudomonas putida S-313. First iron and the cosubstrate alpha-ketoglutarate (KG) coordinate to the enzyme. Second, the alkyl sulfate binds to the active site, displacing a water molecule from the iron center and liberating an unsaturated iron atom. Subsequently a dioxygen molecule binds the iron cation. One oxygen atom of the dioxygen is transferred to KG, yielding succinate and carbon dioxide as products. The iron is thereby oxidized, and a ferryl Fe(IV)=O species is formed, which then hydroxylates the alkyl sulfate via a radical intermediate. Finaly sulfate ion and succinate are released and two water molecules complete the iron coordination sphere. This figure was adapted from the following references (Muller et al, 2004, Biochemistry; Muller et al, 2005, J Biol Chem; Grzyska et al, 2010, Proc Natl Acad Sci USA).
 Family details
Family details